Search results
Search for "supramolecular polymers" in Full Text gives 23 result(s) in Beilstein Journal of Organic Chemistry.
Switchable molecular tweezers: design and applications
Beilstein J. Org. Chem. 2024, 20, 504–539, doi:10.3762/bjoc.20.45

- the two metallic centers in the tweezers cavity in a bridging mode. For the heterometallic Zn/Au system, luminescence quenching was observed in the closed state. The pyridine-hydrazine-pyridine unit has been extensively studied by Lehn and co-workers in coordination-responsive supramolecular polymers
Cyclodextrins as building blocks for new materials
Beilstein J. Org. Chem. 2023, 19, 889–891, doi:10.3762/bjoc.19.66

- science that require high performance with minimal environmental impact. They are involved in the construction of interlocked molecules (rotaxanes and catenanes), supramolecular polymers, artificial enzymes, hydrogels, metal–organic frameworks, supramolecular solvents, fibers, nanotubes, nanoparticles
In-depth characterization of self-healing polymers based on π–π interactions
Beilstein J. Org. Chem. 2021, 17, 2496–2504, doi:10.3762/bjoc.17.166
- of Photonic Technology (IPHT), e. V. Jena, Albert-Einstein-Straße 9, 07745 Jena, Germany 10.3762/bjoc.17.166 Abstract The self-healing behavior of two supramolecular polymers based on π–π-interactions featuring different polymer backbones is presented. For this purpose, these polymers were
- behavior was also quantified in detail using an indenter. Keywords: characterization of polymers; π–π-interactions; self-healing polymers; supramolecular polymers; Introduction Damage inflicted on different materials is omnipresent. Consequently, nature established a mechanism dealing with this problem
- behavior as well as the in-depth characterization of the molecular behavior and the macroscopic properties, which reveals new insights into the self-healing materials based on π–π interactions. Results and Discussion Polymer synthesis For the synthesis of supramolecular polymers based on π-π interactions a
Supramolecular polymers with reversed viscosity/temperature profile for application in motor oils
Beilstein J. Org. Chem. 2021, 17, 105–114, doi:10.3762/bjoc.17.11

- , 64293 Darmstadt, Germany 10.3762/bjoc.17.11 Abstract We report novel supramolecular polymers, which possess a reversed viscosity/temperature profile. To this end, we developed a series of ditopic monomers featuring two self-complementary binding sites, either the guanidiniocarbonyl pyrrole carboxylic
- highly cost effective due to their simple chemical nature. However, this also prevents their further development on a molecular level. For this reason, we set out to develop novel VIIs based on the formation of supramolecular polymers from low-molecular weight building blocks. This would allow for an in
- develop such novel VIIs, we envisaged the use of ditopic building blocks, which are able to reversibly form supramolecular polymers at higher temperatures. Supramolecular polymerization should increase the hydrodynamic radius and thus was expected to lead to increased viscosities [9]. There are different
Supramolecular polymerization of sulfated dendritic peptide amphiphiles into multivalent L-selectin binders
Beilstein J. Org. Chem. 2021, 17, 97–104, doi:10.3762/bjoc.17.10

- of rigid supramolecular polymers. Since multivalent sulfated supramolecular structures mimic naturally occurring L-selectin ligands, the corresponding affinity was evaluated using a competitive SPR binding assay and benchmarked to an ethylene glycol-decorated supramolecular polymer. Keywords: L
- -selectin binders; multivalency; self-assembly in water; supramolecular polymers; Introduction Deciphering the interaction of artificial molecular building blocks with biological components is a key element on the way to understanding and selectively manipulating biological systems. Throughout nature
- polymers as a promising class of synthetic scaffolds [10][11][12][13][14]. Supramolecular polymers do provide additional features like a high degree of flexibility and excellent adaptivity, which are critical in biological interactions [15]. As water is the dominant solvent in biological systems, aqueous
Construction of pillar[4]arene[1]quinone–1,10-dibromodecane pseudorotaxanes in solution and in the solid state
Beilstein J. Org. Chem. 2020, 16, 2954–2959, doi:10.3762/bjoc.16.245

- , including molecular switches, molecular logic gates, molecular machines, supramolecular polymers, etc. [6][7][8][9][10][11]. Pseudorotaxanes not only are used as the supramolecular precursors for the synthesis of rotaxanes and catenanes but also play an important role in the construction of supramolecular
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- have led to applications in molecular recognition, sensing, molecular machines and devices, supramolecular polymers, stimuli-responsive materials, supramolecular catalysis and drug-delivery systems [4][5][6][7]. Inspired by the attractive role of various N-heterocyclic building blocks such as
- imidazoles [8][9], polypyrroles [10][11], and indole moieties [12][13], as part of supramolecular receptors, triazole heterocycles containing macrocycles have recently been introduced as new host molecules for the selective recognition of ions, mechanically interlocked molecules (MIMs), supramolecular
- polymers etc. [14][15]. Noncovalent interactions play a dynamic role in the binding mechanism of triazoles as macrocyclic receptors. It has been reported that the combined effects of both an electron lone pair on the nitrogen of the heterocycle and the acidic C5–H proton make 1,2,3-triazoles interesting
Morphology-tunable and pH-responsive supramolecular self-assemblies based on AB2-type host–guest-conjugated amphiphilic molecules for controlled drug delivery
Beilstein J. Org. Chem. 2019, 15, 1925–1932, doi:10.3762/bjoc.15.188

- HGCMs containing one host and other guest moieties have been used to construct intramolecular complexes [36], cyclic oligomers [37], supramolecular polymers [23][38][39][40], gels [41][42], etc. However, AB-type HGCMs were still limited to obtain non-spherical stimuli-responsive supramolecular self
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
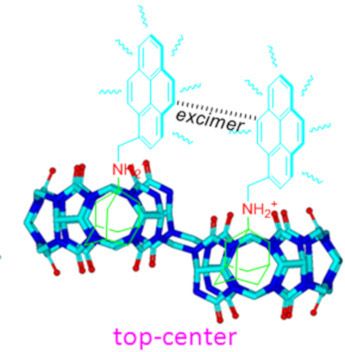
- adamantaneammonium (ADA) or alkylammonium ions into the cavity, forming a ternary complex. The novel binding capacity of NS-CB[10] has been utilized to form supramolecular polymers [24][25][26] and polymer nanoparticles [27]. More importantly, Isaacs et al. discovered that when the unsymmetrical guest ADA molecules
Fabrication, characterization and adsorption properties of cucurbit[7]uril-functionalized polycaprolactone electrospun nanofibrous membranes
Beilstein J. Org. Chem. 2019, 15, 992–999, doi:10.3762/bjoc.15.97

- /nanoreactor and supramolecular polymers, etc. [25][27][28][29][30][31][32]. Unlike other hosts such as CDs, calix[n]arenes, or pillar[n]arenes, the fabrication of CB[n]-functionalized nanofibers by electrospinning is a challenging task due to the poor solubility of CB[n] in common solvents. To develop CB[n
Synthesis and supramolecular properties of regioisomers of mononaphthylallyl derivatives of γ-cyclodextrin
Beilstein J. Org. Chem. 2017, 13, 2509–2520, doi:10.3762/bjoc.13.248
- , Hlavova 8, 128 43, Prague 2, Czech Republic Department of Analytical Chemistry, Faculty of Science, Charles University, Hlavova 8, 128 43, Prague 2, Czech Republic 10.3762/bjoc.13.248 Abstract Monosubstituted derivatives of γ-cyclodextrin (γ-CD) are suitable building blocks for supramolecular polymers
- supramolecular polymers [9] multifunctional molecules are assembled in a regular manner. To avoid branching, monosubstituted CDs are required for a linear supramolecular polymer of CDs with guest groups attached. Monosubstituted derivatives of CDs [10] include three regioisomers, namely 2I-O-, 3I-O-, and 6I-O
- reasons. Compared to α- or β-CD, the number of publications dealing with γ-CD is quite small, e.g., [14][15]. The naphthyl group is well known for its ability to make inclusion complexes with CDs [16]. The allyl connecting linker is relatively rigid and should support the formation of supramolecular
Interactions between shape-persistent macromolecules as probed by AFM
Beilstein J. Org. Chem. 2017, 13, 938–951, doi:10.3762/bjoc.13.95

- macromolecular systems [18]. CDs have already been employed for the construction of supramolecular polymers [19][20][21], supramolecular hydrogels [22][23], molecular printboards [24][25] or multivalent interfaces [26][27][28] with tunable chemical and physical properties. Herein, for the first time, we present
- clear maximum at 63 pN, determined by a Gaussian fit to the distribution, and a weak second maximum at about double this value. We conclude that 63 ± 10 pN is the rupture force for a single bond between our supramolecular polymers 12 established by the ditopic guest 9. The value agrees with rupture
A journey in bioinspired supramolecular chemistry: from molecular tweezers to small molecules that target myotonic dystrophy
Beilstein J. Org. Chem. 2016, 12, 125–138, doi:10.3762/bjoc.12.14

- ] and dendrimers [64][65] – work that ultimately led Cyrus Anderson, Darrell Kuykendall, Ying Li, Taiho Park, Kwansima Quansah, and Mauricio Suarez to a broader range of supramolecular polymers, including liquid crystals [66], network blends [67], alternating copolymers [68], reversible adhesives [69
Supramolecular structures based on regioisomers of cinnamyl-α-cyclodextrins – new media for capillary separation techniques
Beilstein J. Org. Chem. 2016, 12, 97–109, doi:10.3762/bjoc.12.11

- that the position of the cinnamyl group plays an important role in the intermolecular complex formation. Keywords: capillary electrophoresis; cyclodextrin derivatives; mono-cinnamyl; regioisomers; supramolecular structures; Introduction Supramolecular polymers (SP) are aggregates of monomer units
Self-assemblies of γ-CDs with pentablock copolymers PMA-PPO-PEO-PPO-PMA and endcapping via atom transfer radical polymerization of 2-methacryloyloxyethyl phosphorylcholine
Beilstein J. Org. Chem. 2015, 11, 2267–2277, doi:10.3762/bjoc.11.247

- PR supramolecular polymers show potential as dynamic-responsive materials, carriers for controlled drug release, biosensors and catalysts. Experimental Materials PPO-PEO-PPO with a central block of 90 PEO units and two flank blocks of 5 PPO units was supplied by Zhejiang Huangma Chemical Industry
Synthesis, structure, and mechanical properties of silica nanocomposite polyrotaxane gels
Beilstein J. Org. Chem. 2015, 11, 2194–2201, doi:10.3762/bjoc.11.238

- generating systems, nanocomposites should satisfy both a high dielectric constant that is assured by the nanoparticles and a low elastic modulus [7]. Mechanically interlocked supramolecular polymers, such as polyrotaxane [8][9], can control the interface between the matrix polymer and the nanoparticles
Conformational equilibrium in supramolecular chemistry: Dibutyltriuret case
Beilstein J. Org. Chem. 2015, 11, 2105–2116, doi:10.3762/bjoc.11.227
- intramolecular HBing present in some heterocycles results in a molecular geometry suitable for association by multiple hydrogen bonding [11][12][13][14][15][16][17][18][19][20][21][22][23] making possible the formation of, inter alia, stable supramolecular polymers [24][25][26][27][28][29][30]. Such
Supramolecular chemistry: from aromatic foldamers to solution-phase supramolecular organic frameworks
Beilstein J. Org. Chem. 2015, 11, 2057–2071, doi:10.3762/bjoc.11.222

- favorite. Many years later at Fudan, I initiated a project to study the potential of its radical cation stacking in controlling the folded conformation of linear molecules and two- and three-dimensional supramolecular polymers and frameworks. My life in the small town of Odense was also memorable. Its calm
Synthesis and characterization of a new photoinduced switchable β-cyclodextrin dimer
Beilstein J. Org. Chem. 2014, 10, 2874–2885, doi:10.3762/bjoc.10.304

- cavities of cis-AZO-CDim 1 are complexed simultaneously by two adamantyl units of the guest forming a 1:1 complex while trans-AZO-CDim 1 seems to lead to the formation of supramolecular polymers with an n:n stoichiometry. Keywords: azobenzene; cyclodextrins; inclusion complex; photoisomerization
- complex formation through their wider rim with two adamantyl units belonging to two different ADAdim 4 molecules, leading to the formation of supramolecular polymers with an n:n stoichiometry. This situation has already been encountered in the complex of ADAdim 4 and a β-CD dimer bearing a terephthalic
- moiety as the linker [36]. At this stage, based on the molecular dynamics study, at least two supramolecular polymers can be considered: the first is linear, as often described in the literature [43][44] (Figure 8b), and the second is cyclic (Figure 8c). Furthermore, it is possible that such linear or
Superstructures of fluorescent cyclodextrin via click-reaction
Beilstein J. Org. Chem. 2013, 9, 827–831, doi:10.3762/bjoc.9.94

- shape, through poly(host–guest)-interactions, was found. Furthermore, we were able to show the collapse of the supramolecular polymers upon addition of potassium adamantane-1-carboxylate. Experimental General remarks All reagents used were commercially available (Sigma-Aldrich, Acros Organics) and used
Azobenzene dye-coupled quadruply hydrogen-bonding modules as colorimetric indicators for supramolecular interactions
Beilstein J. Org. Chem. 2012, 8, 486–495, doi:10.3762/bjoc.8.55
- desirable because they usually pair with high affinity and high fidelity [12]. High-affinity hydrogen-bonding units have found particular applications in supramolecular polymers [13][14], in which the presence of fewer hydrogen bonds means that the desired assemblies are not achieved, or results in polymers
Chain stopper engineering for hydrogen bonded supramolecular polymers
Beilstein J. Org. Chem. 2010, 6, 869–875, doi:10.3762/bjoc.6.102

- Cedex, France 10.3762/bjoc.6.102 Abstract Supramolecular polymers are linear chains of low molar mass monomers held together by reversible and directional non-covalent interactions, which can form gels or highly viscous solutions if the self-assembled chains are sufficiently long and rigid. The
- obtained when two hydrogen bond acceptors are placed in the same relative position as for the monomer and when no hydrogen bond donor is present. Keywords: chain stopper; gel; hydrogen bond; supramolecular polymer; urea; Introduction Supramolecular polymers are linear chains of low molar mass monomers
- ][9]. Compared to the well-known organogelators formed by the entanglement of usually crystalline fibers [10][11][12][13], supramolecular polymers display some specific features. In particular, hydrogen-bonded supramolecular polymers are often dynamic at room temperature, which means that they do not
Self-association of an indole based guanidinium-carboxylate-zwitterion: formation of stable dimers in solution and the solid state
Beilstein J. Org. Chem. 2010, 6, No. 3, doi:10.3762/bjoc.6.3
- aromatic scaffolds such as benzene or furan instead of pyrrole or with an amidinium cation instead of a guanidinium cation was also confirmed by DFT calculations [14]. Zwitterion 1 has thus found widespread application in the formation of self-assembled nanostructures such as vesicles or supramolecular
- polymers [15][16][17]. We have now synthesized and studied the indole based zwitterion 2, a close analogue of 1. In 2 the guanidinium group is not acylated as in 1 but conjugated to an aromatic ring. Compared to the parent guanidinium cation, in both cases the acidity of the NHs is significantly increased














































































































































































































![[Graphic 1]](/bjoc/content/inline/1860-5397-11-222-i19.png?max-width=637&scale=1.18182) 16 and dynamic [2]catenane formed by compounds 17–19.
16 and dynamic [2]catenane formed by compounds 17–19.






























































